UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
| Date of Report (Date of Earliest Event Reported): | |||||
(Exact name of registrant as specified in its charter)
| (State or other jurisdiction of incorporation) | (Commission File Number) | (I.R.S. Employer Identification No.) | ||||||
| (Address of principal executive offices) | (Zip Code) | |||||||
| Registrant’s telephone number, including area code: | ( | |||||||
Former name or former address, if changed since last report
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) | ||||
| | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) | ||||
| | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) | ||||
| | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) | ||||
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol | Name of each exchange on which registered | ||||||
| N/A | ||||||||
Indicate by check mark whether the registrant is an emerging growth company as defined in as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
| Emerging growth company | | |||||||
| If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. | ☐ | ||||
Item 7.01. Regulation FD Disclosure
On September 22, 2022, INNOVATE Corp. (the “Company”) will be making a presentation to Odeon Capital Group LLC and select investors. A copy of the presentation is attached as Exhibit 99.1 to this Current Report on Form 8-K.
The information set forth in (and incorporated by reference into) this Item 7.01, including Exhibit 99.1 hereto, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1914, as amended (the “Exchange Act”) or otherwise subject to the liabilities of that Section. The information in this Item 7.01,including Exhibit 99.1 hereto, shall not be incorporated by reference into any filings under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such a filing.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits
| Exhibit No. | Description | ||||
| 99.1 | |||||
| 104 | Cover Page Interactive Data File (the cover page XBRL tags are embedded within the inline XBRL document). | ||||
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
Date: September 22, 2022
INNOVATE Corp. (Registrant) | |||||||||||||||||
| By: | /s/ Michael J. Sena | ||||||||||||||||
| Name: Michael J. Sena | |||||||||||||||||
| Title: Chief Financial Officer | |||||||||||||||||

VATE LISTED NYSE

INFRASTRUCTURE • DBM Global: Largest steel fabrication and erection company in the USA LIFE SCIENCES • R2 Technologies: the first and only device to employ Cryomodulationtm using PRECISION COOLING to address SOURCES of redness, benign lesions, inflammation and more • MediBeacon: Developing the first real-time monitoring of kidney function, which has received “breakthrough device designation” from the FDA and is being developed to address a $7 billion(1) market SPECTRUM • HC2 Broadcasting: One of the largest broadcast station groups in the US, owning 2.3 billion MHz POPs of spectrum INNOVATE is a platform of best-in-class assets in 3 key areas of the new economy: 2 (1) Based on studies from LifeSci Advisors (2019) and L.E.K. (2017) commissioned by MediBeacon

INFRASTRUCTURE 3

• The largest steel fabrication and erection company in the USA • Approx. $1.5 billion Backlog as of 6/30/22 (down from $1.6 billion on 12/31/21) Approx. $1.7 billion Adjusted Backlog(1) as of 6/30/22 taking into account awarded, but not yet signed contracts • DBM is poised for growth and its performance is underscored by its ability to sustain backlog levels, deliver revenue growth and maintain a robust pipeline • DBM’s best in class management team has a proven long-term track record of executing complex projects • DBM expanded its geographic reach in 2021 with its acquisition of Banker Steel • DBM Vircon is a construction technology company providing state of the art modeling, detailing and digital engineering for high-profile projects internationally • DBM’s GrayWolf subsidiary provides service and maintenance for power, petrochemical, refinery and pulp/paper markets DBM Global 4 (1) Adjusted Backlog takes into consideration awarded, but not yet signed contracts and there are no assurances that these contracts will be entered into in the timeframe we anticipate or at all.

SoFi Stadium, Los Angeles Hudson Yards, New YorkApple Headquarters Facebook MPK21 Phoenix Convention CenterFort Benning Hospital Cosmopolitan Resort & Casino 5 Select DBM Steel Fabrication & Erection Projects 5

DBM Second Quarter 2022 Segment Highlights 6 ■ Address working capital needs from growth, changes in projects and timing ■ Strong backlog provides runway for future cash generation of the business ■ Revenue and EBITDA increases primarily due to the acquisition of Banker Steel which was acquired May 27, 2021 ■ Point of sale margins on projects were under pressure through the 1st half of 2021 as a result of the pandemic ■ 2022 margins reflect burn-off of backlog sold in the 1st half of 2021 and prior as projects are completed 12 to 18 months after contacts are signed into backlog ■ Management has seen improvements in point-of-sale margins beginning in the 2nd half 2021 ■ 2021 began to also see larger, more complex project entering the market (LA Clippers Arena, JFK Airport) ■ Reported backlog level of $1.5B ■ Taking into consideration awarded but not yet signed contracts, adjusted backlog was ~$1.7B Financials ($ millions) 2021 LTM 6/30/22 Revenue $ 1,159.7 $ 1,550.7 Net Income $ 16.9 $ 28.4 Adjusted EBITDA (1) $ 78.4 $ 94.6 (1) See Appendix for reconciliation of Non-GAAP to U.S. GAAP. (2) All data as of June 30, 2022 unless otherwise noted. ~$1,717.8 Overview Near-Term Focus ($ millions) Trending Backlog

PANSEND GENOVEL 7 LIFE SCIENCES

• Pansend is INNOVATE’s life sciences platform • Pansend has investments in four life science companies: R2 Technologies, MediBeacon, Triple Ring Technologies and Genovel Orthopedics • Pansend was founded by and is led by David A. Present, MD and Cherine Eldumiati Plumaker. Their outstanding leadership has produced a proven track record for developing and monetizing life science companies • In 2018, Pansend sold BeneVir to Janssen Biotech (J&J) for up to $1 billion(1). BeneVir’s proprietary T-Stealth oncolytic virus platform develops oncolytic viruses used to infect and destroy cancer cells • INNOVATE receives 75% of Pansend’s economic distributions after receipt of INNOVATE’s invested capital, plus a hurdle rate Pansend Life Sciences, LLC 8 (1) Subject to certain pre-determined milestones. There are no assurances that milestones will be achieved.

• Glacial Rx®, launched in 2021 by R2 Technologies, is the first and only device to employ Cryomodulationtm using PRECISION COOLING to address SOURCES of redness, benign lesions, inflammation and more. • Massive market opportunity: $22 billion(1) skin tone evening, lightening and brightening products global market • Glacial Rx® was developed by Drs. Rox Anderson, Dieter Manstein and Henry Chan – world famous dermatologists at Harvard Medical School who have created numerous commercially successful aesthetic dermatological devices such as Laser Hair Removal, Fractional Lasers/Fraxel, and Coolsculpting • Glacial Rx® was named among “2021 Launches Doctors are Buzzing About”(2) • Glacial Spa® launched in China in the first quarter 2022. Glacial AI® (an autonomous robot) is expected to launch in 2024 • Strong patent portfolio: 105 issued patents & 46 patents pending R2 Technologies 9 (1) Source: The Jarvis Report 2016 (R2-Commissioned) and “Revolution in Aesthetic Medicine” (11-21-2015) (2) https://www.realself.com/news/best-beauty-launches-for-2021

10

MediBeacon 11 (1) Based on studies from LifeSci Advisors (2019) and L.E.K. (2017) commissioned by MediBeacon (2) Based on Effimed Exploratory GI Market Research (2017) commissioned by MediBeacon (3) Based on Medicare Plan B data from 2012-2017 combined with MediBeacon market assumptions (4) Based on US Centers for Disease and Prevention (CDC) statistics, “Urologic Complications from Surgery” Washington University School of Medicine (2021) and MediBeacon market assumptions • MediBeacon has developed the first real-time monitoring of kidney function which has received “breakthrough device designation” from the FDA and is being developed to address a $7 billion market(1). According to the FDA, a “Breakthrough Device” like the Transdermal GFR Measurement System is a product that has the potential to be more effective at diagnosing a life threatening or irreversible debilitating disease or condition compared to the current standard of care • Significant medical opinions exist that current diagnostic tests to measure kidney function are inaccurate, providing indirect measurements, and are not real-time. Accurate kidney measurement is critical for treating patients in the hospital with 20-40% of patients at risk for kidney failure in critical care settings • MediBeacon’s Transdermal GFR Measurement System includes a patented compound, Lumitrace™ that is entirely eliminated by the kidneys. Lumitrace™ fluorescence is detected by a sensor placed on the skin, analogous to pulse oximetry for oxygen saturation. • MediBeacon’s technology platform is being developed for use in gastrointestinal, ocular, and surgical visualization markets. Gastrointestinal is a $1 billion market(2), ocular is a $200 million market(3) and surgical visualization is a $100 million market(4) • The FDA accepted MediBeacon Inc.'s ("MediBeacon") Investigational Device Exemption (IDE) application and, on June 26, 2022, MediBeacon initiated its Transdermal GFR Measurement System FDA Pivotal Study. • Strong patent portfolio: 164 granted global patents, 46 granted U.S. patents & 212 global pending applications

12

Triple Ring Technologies • A diversified, profitable technology and product development firm with 100 experienced scientists & engineers, including 21 PhDs • Completed 450 projects from technology assessments to product development • 2021 revenue was $28 million • Pansend owns 23%(1) Genovel Orthopedics • “Mini Knee” for early osteoarthritis of the knee & “Anatomical Knee” a novel total knee replacement that has been granted a US patent • Invented by Dr. Peter Walker (creator of the original Total Knee) at NYU Department of Orthopedic Surgery • Pansend owns 75%(1) Other Pansend Opportunities 13 (1) On a fully diluted basis.
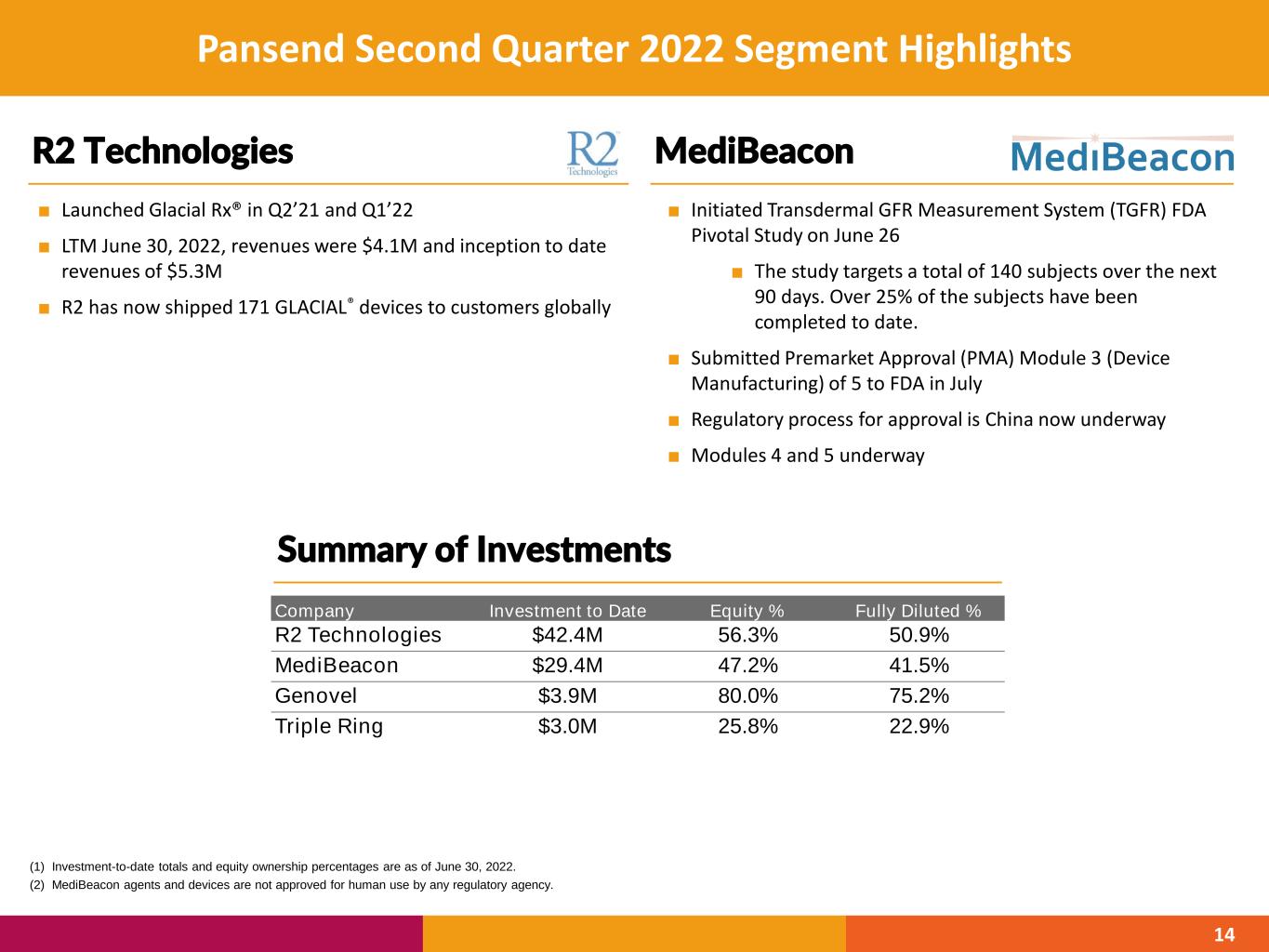
Pansend Second Quarter 2022 Segment Highlights 14 MediBeaconR2 Technologies (1) Investment-to-date totals and equity ownership percentages are as of June 30, 2022. (2) MediBeacon agents and devices are not approved for human use by any regulatory agency. Company Investment to Date Equity % Fully Diluted % R2 Technologies $42.4M 56.3% 50.9% MediBeacon $29.4M 47.2% 41.5% Genovel $3.9M 80.0% 75.2% Triple Ring $3.0M 25.8% 22.9% ■ Initiated Transdermal GFR Measurement System (TGFR) FDA Pivotal Study on June 26 ■ The study targets a total of 140 subjects over the next 90 days. Over 25% of the subjects have been completed to date. ■ Submitted Premarket Approval (PMA) Module 3 (Device Manufacturing) of 5 to FDA in July ■ Regulatory process for approval is China now underway ■ Modules 4 and 5 underway Summary of Investments ■ Launched Glacial Rx® in Q2’21 and Q1’22 ■ LTM June 30, 2022, revenues were $4.1M and inception to date revenues of $5.3M ■ R2 has now shipped 171 GLACIAL® devices to customers globally

SPECTRUM HC2 Broadcasting 15

• One of the largest broadcast station groups in the US with 249 operating broadcast stations in over 100 markets – including 33 of the top 34 markets • One of the largest owners of broadcast spectrum in the US with more than 2.3 billion MHz POPs of spectrum • As cable TV “cord cutting” increases and the number of over-the-air (OTA) viewers continues to grow – HC2 Broadcasting has one of the largest OTA distribution platform in the US • Broadcasting’s stations carry approx. 1,500 channels with programming from over 70 diginets • Rising demand for a national distribution platform by large content providers increases revenues and margins for HC2 Broadcasting • ATSC 3.0 (Next Gen TV) technology creates additional revenue opportunities • Received an experimental license grant (File Number 1307-EX-ST-2022) from the FCC’s Office of Engineering and Technology for a trial in Fort Wayne Indiana using ATSC 3.0 • Recently received approval of ATSC 3.0 applications for 2 Fort Wayne Station as part of the trial HC2 Broadcasting 16

17 HC2’s stations carry approx. 1,500 channels with programming from over 70 diginets

HC2 Broadcasting Second Quarter 2022 Segment Highlights 18 ■ Finished all station builds targeted in 2022 ■ Completed the build-out of a new station for license WKOB-LD in New York City. The new site for the station is One World Trade Center and is now on-air, providing a far-ranging robust signal to the New York City market ■ Broadcasting will own and operate 249 stations that cover 105 DMAs ■ Broadcasting growth slowed due to softness in advertising market and the origination of new diginets ■ Network business, Azteca America, challenged by the current advertising markets along with declining viewership and increased costs for content Financials ($ millions) 2021 LTM 6/30/22 Station Group $ 18.8 $ 19.2 Network ("Azteca") 23.2 20.6 Revenue $ 42.0 $ 39.8 Net (Loss) $ (12.9) $ (16.5) Adjusted EBITDA (1) $ 6.9 $ 5.1 Overview Near-Term Focus ■ Continue business development and sign up large content providers; strong pipeline of pending lease agreements or revenue shares across multiple markets ■ Seeing new revenue opportunities with shopping networks, news networks and religious networks ■ Explore ATSC 3.0 technologies that offer expanded capability and use of Broadcasting's spectrum Station Growth (1) See Appendix for reconciliation of Non-GAAP to U.S. GAAP.

INNOVATE Summary 19 INFRASTRUCTURE Integrated structural steel and construction services 91% Owned LIFE SCIENCES Develops innovative healthcare technologies to address unmet needs with four investments SPECTRUM One of the largest over-the-air broadcast groups in the U.S. 98% Owned (1) Based on latest fundraising round (2) INNOVATE invested $15M at $135M pre-money valuation on 7/20/21 (3) 3rd Party Investment at $300M pre-money valuation on 7/31/19 (4) As of 6/30/22 Additional Value • In the process of selling 19% in HMN Technologies, and expecting approximately $32M in net proceeds • Sale of BeneVir by Pansend in 2018 includes $512M of potential milestone payments Q2 Update • $1.5B Backlog 6/30/22 • $1.7B Adjusted Backlog(2) 6/30/22 • Robust sales into backlog continue • Awarded JFK project • Point of sale margins continue to improve • Working capital increases have put pressure on cash Q2 Update R2 • Glacial Rx (Q2’21 Launch) and Glacial Spa (Q1’22 Launch) Continue to build sales momentum Medibeacon • Commenced final pivotal study • Submitted 3 of 5 Modules to the FDA • Modules 4 and 5 underway Q2 Update • Continue to drive sales of available capacity on the broadcasting platform • Network business under pressure from market conditions • Exploring next gen revenue opportunities for ATSC 3.0 (1) See Appendix for reconciliation of Non-GAAP to U.S. GAAP. (2) Adjusted Backlog takes into consideration awarded, but not yet signed contracts (1) LTM June 2022 Revenue $1,550.7M Net Income $28.4M Adjusted EBITDA $94.6M (1) Investment Diluted Ownership(4) Most Recent Valuation (1) R2 Technologies $42.4M 50.9% $135M (2) MediBeacon $29.4M 41.5% $300M (3) Genovel $3.9M 75.2% N/A TripleRing Technologies $3.0M 22.9% N/A LTM June 2022 Revenue $39.8M Net Loss $(16.5)M Adjusted EBITDA $5.1M

20 APPENDIX

(1) Net Income attributable to INNOVATE Corp. (2) See Appendix for reconciliation of Non-GAAP to U.S. GAAP (3) Adjusted Backlog takes into consideration awarded, but not yet signed contracts Q2 2022 Financial Highlights 21 Consolidated Q2 Results ■ Revenue increased $148.4M or 60.9% driven by our Infrastructure segment, due primarily to DBM's acquisition of Banker Steel on May 27, 2021, and increases in the Infrastructure market demand along with larger projects entering the market ■ Net Loss attributable to INNOVATE Corp. of $12.4M ■ Adjusted EBITDA(2) increased by $5.6M to $12.1M driven by contribution from Banker Steel at Infrastructure Infrastructure ■ Net Income of $6.8M(1) ■ $20.9M in Adjusted EBITDA (2); contracted backlog of $1.5B (Adjusted ~$1.7B(3)), compared to $1.6B at 12/31/21 Spectrum ■ Net Loss of $5.7M(1) ■ $0.4M in Adjusted EBITDA(2) due to advertising softness, reduced footprint, along with higher costs at the network business Life Sciences ■ Revenue of $1.0M driven by R2’s Glacial Rx and Spa devices Non-operating Corporate ■ Recurring SG&A down $2.3M year-over-year due to accrued settlement with former CEO in prior year Revenue ($ millions) 2Q22 2Q21 Infrastructure $ 382.1 $ 232.0 Life Sciences 1.0 1.2 Spectrum 9.1 10.6 Consolidated INNOVATE $ 392.2 $ 243.8 Net Income (loss) Attrib. to INNOVATE Corp. & Adj. EBITDA 2Q22 2Q21 ($ millions) NI(1) Adj. EBITDA(2) NI(1) Adj. EBITDA(2) Infrastructure $ 6.8 $ 20.9 $ 1.4 $ 13.9 Life Sciences (5.3) (7.5) (4.3) (6.1) Spectrum (5.7) 0.4 (1.1) 2.7 Non-operating Corporate (9.5) (3.4) (19.2) (5.7) Other & Eliminations 1.3 1.7 1.2 1.7 Consolidated INNOVATE, Excluding Disc Ops $ (12.4) $ 12.1 $ (22.0) $ 6.5 Discontinued Operations $ — $ (1.5) Net (loss) Attrib. to INNOVATE Corp. $ (12.4) $ (23.5)

Current Credit Picture 22 ($ millions) (1) Debt Maturity Profile excludes Preferred Stock and capital leases (2) Infrastructure Line of Credit reflects maturity in 2024 and not U.S. GAAP presentation (3) Debt Amortization and Maturity Profile chart presents debt annual amortization and maturity payments. $0.0 $50.0 $100.0 $150.0 $200.0 $250.0 $300.0 $350.0 $400.0 $450.0 $500.0 2022 2023 2024 2025 2026 Debt Amortization and Maturity Profile(3) Holdco Infrastructure Spectrum Life Science Debt Summary(1) ($ millions) Maturity Jun-22 Dec-21 8.50% Senior Secured Notes 2026 $ 330.0 $ 330.0 7.50% Convertible Senior Notes 2022 ---- 3.2 7.50% Convertible Senior Notes 2026 51.8 51.8 Line of Credit 2024 5.0 5.0 Infrastructure Debt(2) Various 230.4 188.6 Spectrum Debt 2022 52.2 52.2 Life Science Debt 2022 0.5 ---- Total Principal Outstanding $ 669.9 $ 630.8 Unamortized OID and DFC (2.8) (4.5) Total Debt $ 667.1 $ 623.3 Cash & Cash Equivalents 24.9 45.5 Net Debt $ 642.2 $ 580.8 $61.8 $18.3 $120.8 $7.2 $461.8

Reconciliation of U.S. GAAP Income (Loss) to Adjusted EBITDA 23 (in millions) Three Months ended June 30, 2022 Infrastructure Life Sciences Spectrum Non-operating Corporate Other and Elimination INNOVATE Net (loss) attributable to INNOVATE Corp. $ (12.4) Less: Discontinued operations — Net Income (loss) attributable to INNOVATE Corp., excluding discontinued operations $ 6.8 $ (5.3) $ (5.7) $ (9.5) $ 1.3 $ (12.4) Adjustments to reconcile net income (loss) to Adjusted EBITDA: Depreciation and amortization 5.3 — 1.5 0.1 — 6.9 Depreciation and amortization (included in cost of revenue) 3.6 — — — — 3.6 Other operating expense — — 1.7 — — 1.7 Interest expense 2.2 — 1.9 8.4 — 12.5 Other (income) expense, net (1.4) (0.2) 1.4 (1.2) — (1.4) Gain on sale or dissolution of subsidiary — — — (0.1) — (0.1) Income tax expense (benefit) 3.5 — — (1.5) — 2.0 Noncontrolling interest 0.7 (2.1) (0.5) — 0.4 (1.5) Share-based compensation expense — 0.1 — 0.4 — 0.5 Nonrecurring Items 0.1 — — — — 0.1 Acquisition and disposition costs 0.1 — 0.1 — — 0.2 Adjusted EBITDA $ 20.9 $ (7.5) $ 0.4 $ (3.4) $ 1.7 $ 12.1

Reconciliation of U.S. GAAP Income (Loss) to Adjusted EBITDA 24 (in millions) Three Months ended June 30, 2021 Infrastructure Life Sciences Spectrum Non-operating Corporate Other and Elimination INNOVATE Net (loss) attributable to INNOVATE Corp. $ (23.5) Less: Discontinued operations (1.5) Net Income (loss) attributable to INNOVATE Corp., excluding discontinued operations $ 1.4 $ (4.3) $ (1.1) $ (19.2) $ 1.2 $ (22.0) Adjustments to reconcile net income (loss) to Adjusted EBITDA: Depreciation and amortization 3.3 0.1 1.4 — — 4.8 Depreciation and amortization (included in cost of revenue) 2.7 — — — — 2.7 Other operating (income) — — (0.2) — — (0.2) Interest expense 2.2 — 2.4 7.8 — 12.4 Loss on early extinguishment or restructuring of debt 1.5 — — 0.1 — 1.6 Other (income) expense, net (4.1) — 0.4 3.3 — (0.4) Income tax expense 1.2 — — 1.4 — 2.6 Noncontrolling interest 0.2 (1.9) (0.5) — 0.6 (1.6) Share-based compensation expense — — 0.2 0.5 — 0.7 Nonrecurring Items 0.2 — — — — 0.2 COVID-19 Costs 4.0 — — — — 4.0 Acquisition and disposition costs 1.3 — 0.1 0.4 (0.1) 1.7 Adjusted EBITDA $ 13.9 $ (6.1) $ 2.7 $ (5.7) $ 1.7 $ 6.5

Reconciliation of U.S. GAAP Income (Loss) to Adjusted EBITDA 25 (in millions) DBM Global (Infrastructure Segment) Year Ended December 31, 2021 Six Months ended June 30, 2021 Six Months ended June 30, 2022 Last Twelve Months ended June 30, 2022 Net Income attributable to INNOVATE Corp., excluding discontinued operations $ 16.9 $ 1.4 $ 12.9 $ 28.4 Adjustments to reconcile net income (loss) to Adjusted EBITDA: Depreciation and amortization 19.1 5.7 10.6 24.0 Depreciation and amortization (included in cost of revenue) 12.2 5.0 7.3 14.5 Other operating (income) expense 0.4 - (0.6) (0.2) Interest expense 8.5 4.1 4.4 8.8 Other (income) expense, net (4.0) (3.9) (1.3) (1.4) Loss on early extinguishment of debt 1.5 1.5 - - Income tax expense 10.5 1.2 6.4 15.7 Noncontrolling interest 1.8 0.2 1.3 2.9 Nonrecurring Items 0.5 0.4 0.1 0.2 COVID-19 costs 8.6 7.9 - 0.7 Acquisition and disposition costs 2.4 1.7 0.3 1.0 Adjusted EBITDA $ 78.4 $ 25.2 $ 41.4 $ 94.6
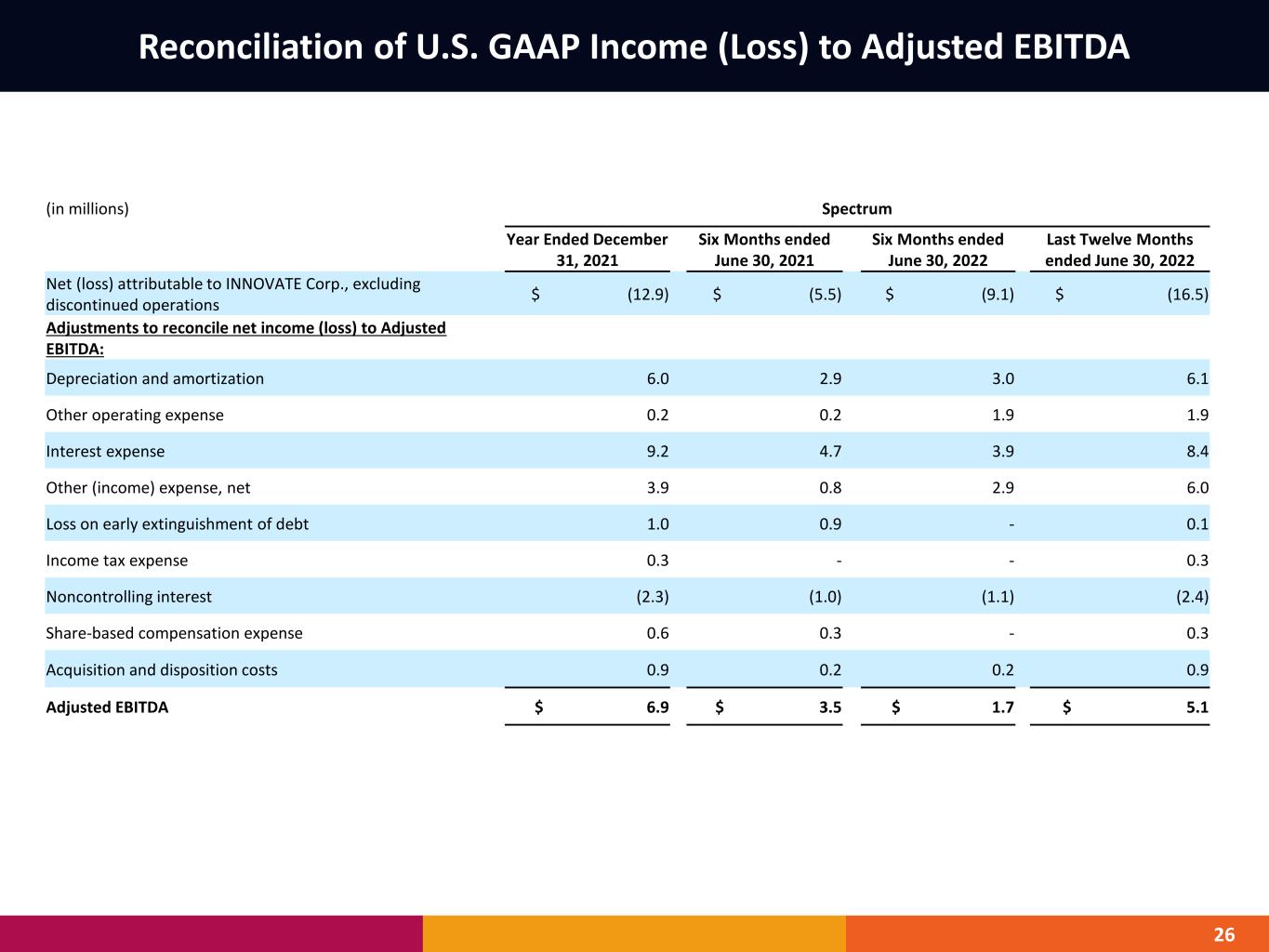
Reconciliation of U.S. GAAP Income (Loss) to Adjusted EBITDA 26 (in millions) Spectrum Year Ended December 31, 2021 Six Months ended June 30, 2021 Six Months ended June 30, 2022 Last Twelve Months ended June 30, 2022 Net (loss) attributable to INNOVATE Corp., excluding discontinued operations $ (12.9) $ (5.5) $ (9.1) $ (16.5) Adjustments to reconcile net income (loss) to Adjusted EBITDA: Depreciation and amortization 6.0 2.9 3.0 6.1 Other operating expense 0.2 0.2 1.9 1.9 Interest expense 9.2 4.7 3.9 8.4 Other (income) expense, net 3.9 0.8 2.9 6.0 Loss on early extinguishment of debt 1.0 0.9 - 0.1 Income tax expense 0.3 - - 0.3 Noncontrolling interest (2.3) (1.0) (1.1) (2.4) Share-based compensation expense 0.6 0.3 - 0.3 Acquisition and disposition costs 0.9 0.2 0.2 0.9 Adjusted EBITDA $ 6.9 $ 3.5 $ 1.7 $ 5.1

For more information email ir@innovatecorp.com or visit www.innovatecorp.com 27

Safe Harbor Statement under the Private Securities Litigation Reform Act of 1995: This presentation contains, and certain oral statements made by our representatives from time to time may contain, "forward-looking statements." Generally, forward-looking statements include information describing actions, events, results, strategies and expectations and are generally identifiable by use of the words “believes,” “expects,” “intends,” “anticipates,” “plans,” “seeks,” “estimates,” “projects,” “may,” “will,” “could,” “might,” or “continues” or similar expressions. Such forward-looking statements are based on current expectations and inherently involve certain risks, assumptions and uncertainties. INNOVATE Corp. (“INNOVATE”) believes these judgments are reasonable, but you should understand that these statements are not guarantees of performance, results or the creation of stockholder value and INNOVATE’s actual results could differ materially from those expressed or implied in the forward-looking statements due to a variety of important factors, both positive and negative, including those that may be identified in subsequent statements and reports filed with the Securities and Exchange Commission (“SEC”), including in our reports on Forms 10-K, 10-Q, and 8-K. Such important factors include, without limitation: developments relating to on-going hostilities in Ukraine, the severity, magnitude and duration of the COVID-19 pandemic, including impacts of the pandemic and of businesses’ and governments’ responses to the pandemic on INNOVATE’s operations and personnel, and on commercial activity and demand across our businesses, capital market conditions, including the ability of INNOVATE and INNOVATE’s subsidiaries to raise capital; the ability of INNOVATE’s subsidiaries and portfolio companies to generate sufficient net income and cash flows to make upstream cash distributions; volatility in the trading price of INNOVATE common stock; the ability of INNOVATE and its subsidiaries and portfolio companies to identify any suitable future acquisition or disposition opportunities; our ability to realize efficiencies, cost savings, income and margin improvements, growth, economies of scale and other anticipated benefits of strategic transactions; difficulties related to the integration of financial reporting of acquired or target businesses; difficulties completing pending and future acquisitions and dispositions; effects of litigation, indemnification claims, and other contingent liabilities; changes in regulations and tax laws; covenants noncompliance risk; interest rate environment; ability to remain in compliance with NYSE listing requirement and risks that may affect the performance of the operating subsidiaries and portfolio companies of INNOVATE. Although INNOVATE believes its expectations and assumptions regarding its future operating performance are reasonable, there can be no assurance that the expectations reflected herein will be achieved. These risks and other important factors discussed under the caption “Risk Factors” in our most recent Annual Report on Form 10-K filed with the SEC, and our other reports filed with the SEC could cause actual results to differ materially from those indicated by the forward-looking statements made in this presentation. You should not place undue reliance on forward-looking statements. All forward-looking statements attributable to INNOVATE or persons acting on its behalf are expressly qualified in their entirety by the foregoing cautionary statements. All such statements speak only as of the date made, and unless legally required, INNOVATE undertakes no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise. 28

Non-GAAP Financial Measures In this presentation, INNOVATE refers to certain financial measures that are not presented in accordance with U.S. generally accepted accounting principles (“GAAP”), including Adjusted EBITDA, which excludes results for discontinued operations, and Adjusted EBITDA for its operating segments. Adjusted EBITDA Adjusted EBITDA is not a measurement recognized under U.S. GAAP. In addition, other companies may define Adjusted EBITDA differently than we do, which could limit its usefulness. Management believes that Adjusted EBITDA provides investors with meaningful information for gaining an understanding of our results as it is frequently used by the financial community To provide insight into an organization’s operating trends and facilitates comparisons between peer companies, since interest, taxes, depreciation, amortization and the other items listed in the definition of Adjusted EBITDA below can differ greatly between organizations as a result of differing capital structures and tax strategies. Adjusted EBITDA can also be a useful measure of a company’s ability to service debt. While management believes that non-U.S. GAAP measurements are useful supplemental information, such adjusted results are not intended to replace our U.S. GAAP financial results. Using Adjusted EBITDA as a performance measure has inherent limitations as an analytical tool as compared to net income (loss) or other U.S. GAAP financial measures, as this non-GAAP measure excludes certain items, including items that are recurring in nature, which may be meaningful to investors. As a result of the exclusions, Adjusted EBITDA should not be considered in isolation and does not purport to be an alternative to net income (loss) or other U.S. GAAP financial measures as a measure of our operating performance. Adjusted EBITDA excludes the results of operations and any consolidating eliminations of our previous Insurance segment. The calculation of Adjusted EBITDA, as defined by us, consists of Net income (loss) as adjusted for discontinued operations; depreciation and amortization; Other operating (income) expense, which is inclusive of (gain) loss on sale or disposal of assets, lease termination costs, asset impairment expense and FCC reimbursements; interest expense; loss on early extinguishment or restructuring of debt; other (income) expense, net; gain on sale or dissolution of subsidiary; income tax expense (benefit); noncontrolling interest; share-based compensation expense; non-recurring items; costs associated with the COVID-19 pandemic and acquisition and disposition costs. Third Party Sources Third party information presented in this presentation is based on sources we believe to be reliable, however there can be no assurance information so presented will prove accurate in whole or in part. 29
